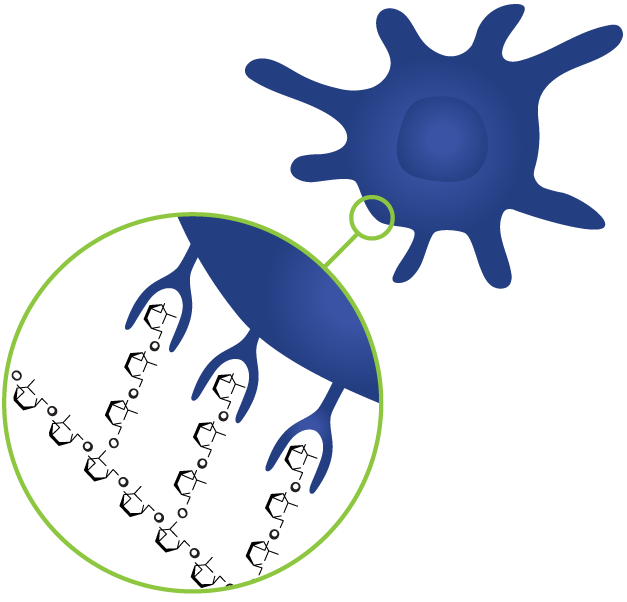
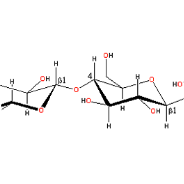
Patent: Therapy enhancing glucan in Neuroblastoma
N. V. Cheung et. al. 2012.A therapeutic composition for treatment of cancer in mammal is disclosed. The composition comprises an effective amount of yeast beta-glucan composition which is suitable for oral administration and for absorption through the gastrointestinal tract of the mammal. The above therapeutic composition ma...
Continue reading